Introduction
“He has created the heavens and the earth in accordance with the requirements of wisdom. Exalted is He above all that they associate with Him.”
Holy Quran 16:4
Our universe is remarkable. It is filled with immense displays of cosmic beauty, from the nebulae that give birth to stars, to the end of stars in dazzling supernovae. Yet by far the most remarkable fact of our universe is the existence of life itself. Our consciousness, born from the loins of the universe, is able to speculate on its origin and ponder on its meaning.
While many count the existence of life and consciousness as something akin to a miracle, there is a deeper miracle behind the scenes. For life to have begun at all, it required certain pre-requisites. The physics of the universe had to take on a certain character for intelligent life to ever develop. Without such characteristics, our universe would be dead and dumb, never seen and never heard. The special nature of the universe includes its initial state in the Big Bang, the laws of the universe, and the actual values of the physical constants by which natural laws work.
With even small changes in any of these domains, the universe would look very different. Some of these alternative universes would collapse quickly, some would be emptier, some wouldn’t have stars. Such universes are entirely possible — there is no physical reason as to why they couldn’t exist. But none of them would harbour life. Biological life requires a specific stage to take place on. If the stage is taken away, the actors aren’t able to perform. Let’s visit this stage, and examine it for ourselves.
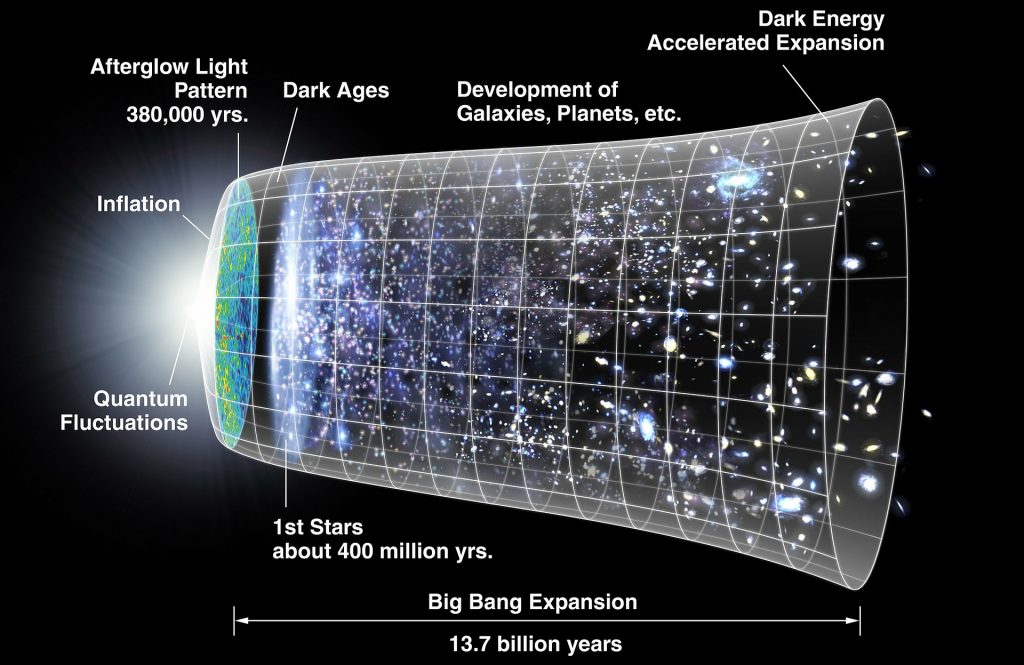
Initial State of the Big Bang
Cosmic energy density — Ω
In the first moments after the Big Bang, the universe had what is known as a global cosmic energy density. This value, denoted by the Greek letter Ω [omega] denotes how tightly packed the universe was in its early state. The value of this density had to be extremely close to 1, or there would be serious consequences. If the density was very slightly lower, the universe would expand so quickly that stars and galaxies would not have formed. If it was slightly larger, the universe would quickly collapse, not allowing stars, galaxies, and planets to arise. As fortune would have it, the universe’s density was almost exactly one, to 60 decimal places. [i] Without this, we would have no stable solar systems and no life.
Barbara Ryden, the author of the textbook Introduction to Cosmology (2003) comments on this precision with amazement:
“The number 10-60 [i.e.: 60 decimal places] is, of course, very tiny. To use an analogy, to change the Sun’s mass by one part in 10-60, you would have to add or subtract two electrons. Our very existence depends on the fantastically close balance between the actual density and the critical density in the early universe… a coincidence at the level of one part in 1060 is extremely far-fetched.” [ii]
Entropy
Then take the universe’s initial state of entropy. Entropy is a measure of the free and potentially useful energy in a system. If a system has a state of low entropy, it means that there is the possibility for energy to flow in ways that makes things happen. Things like stars making essential elements, planets forming, and life living. On the other hand, a system with high entropy has all its useful energy trapped in one form, like a locked treasure chest that needs to be prised open with great difficulty. In the universe, the equivalent of such treasure chests are the iron cores of burnt-out stars. The energy trapped in there can’t be easily extracted. The keys have been thrown away, and the energy is never to be seen again.
The next thing to know about entropy is that it never decreases. In fact, as time goes on, the entropy in the universe is always increasing. This means that more and more energy is getting trapped into these universal treasure chests. and there is less and less free energy to get to work.
Given this, it’s quite the stroke of luck that our universe began in a state of extremely low entropy. If it didn’t, life simply wouldn’t exist – there wouldn’t be enough energy to get things running. [iii]
This arrangement was by no means inevitable. Of all the possible levels of entropy that the universe could have started out with, such a low level was extremely unlikely. In fact, famed English physicist Roger Penrose once calculated how unlikely it was for our universe to have had such an ideal, low entropy initial state. He compared its actual state to much more likely, higher entropy states. He came to the conclusion that the probability of our current arrangement was:
Penrose, amazed at his own findings, did not hold back about the implications of this finding:
“People say ‘well you don’t want such-and-such a theory… because that requires ‘fine-tuning’ or something like that.’ But there’s got to be fine-tuning! This is fine-tuning. This is incredible precision in the organisation of the initial universe.”
Life-Giving Laws
Natural law itself is a marvel. According to our most advanced physics, gravity, electromagnetism, and the ‘strong’/’weak’ nuclear forces allow the organisation of matter into highly complex forms. We know that the first three at least are necessary for life to exist:
“Turn off gravity, and there is nothing to drive matter to collapse into galaxies, stars and planets, or indeed any structure. Turn off electromagnetism, and there is no chemistry as there is nothing to keep electrons bound to nuclei. Turn off the strong force, and there are no nuclei, and so again chemistry is doomed.” [iv]
If we didn’t have these forces, it’s clear that biological life would not exist. Those universes would be cold, dark, and lifeless. Such universes are entirely physically possible — we can model them with ease on supercomputers. The fact that we have these combinations of laws is yet another stroke of good fortune.
Finely-Tuned Constants
These laws don’t operate blindly, but with precision. There are myriad values, or constants, by which natural laws operate. Just as the initial state of the universe had to be finely-tuned, we have discovered that these constants must exist in very narrow margin for biological life to arise and thrive. The required precision can be likened to the engineering process for space craft. If a machine cuts a metal panel even slightly off, or doesn’t screw a bolt in tightly enough, the entire rocket will be in danger. If things are even slightly off, the whole thing is blown out of the sky in front of everyone’s eyes.
The fine-tuning of the universe of the laws of nature is just like this. Laws exist, but they must operate at very specific levels. An attractive force must be this attractive, but not that attractive. A repulsive force must repulse only this much, but no further.
Gravity
Let’s first look at gravity. It is essential for the formation of stars. Stars act as factories for the heavy elements we need for life. When stars explode in supernovae, they seed the universe with the basic elements necessary for life to develop. They also act as central points for planets to gather around, stabilise in orbits, and produce life-permitting atmospheres.
But the right kind of stars require a very specific strength of gravity, denoted by N. If gravity was slightly stronger, stars and planets would be much smaller, and galaxies would be much denser. This would mean stellar orbits would be much more unstable, and planet-crashes would be more commonplace. (Imagine the insurance claims!). Even if a solar system got lucky and its planets had stable orbits, a typical star would only live for 10,000 years, too short a period to allow atmospheres to form, chemistry to fizz and boil, and biology to bubble up. [v]
If gravity was weaker however, the universe would be much colder — it would be much more difficult for stars to form. Those that did form would be unlikely to explode in supernovae. They would simply fizzle out, perhaps forming a black hole in the process. This means the essential life-giving material – carbon and oxygen in particular – would not be expelled, and life wouldn’t have a chance. [vi]

The Strong Force and the Weak Quarks
Other finely-tuned parameters concern two fundamental constituents of life – carbon, and oxygen. Water (H20) requires oxygen, while carbon is the basis of organic chemistry.
The production of carbon in the furnace of stars is famously tricky. It is produced when helium and beryllium come together under immense pressure. When Fred Hoyle, the famed astrophysicist, investigated this phenomenon in post-war England, he was baffled. By rights, as soon as carbon was formed, it should have ripped apart again into different elements. In order to stay stable, and stay carbon, the nucleus would have to vibrate and oscillate at a very specific energy level. Hoyle suggested the resonance was at such a specific level – and was proved right. This energy level, called a resonance, is something that must be inherent in a carbon nucleus. In fact, it must be built-in to the laws of nature itself. Were the resonance level of carbon adjusted by 4% up or down, its production would be jeopardised. [vii]
That is not all. Oxygen has a resonance level delicately placed so that it is produced alongside with carbon. The two are produced together. So not only has carbon a finely-tuned resonance level, oxygen does too. [viii]
Hoyle was candid about his astonishment at finding that these resonances were exactly where they needed to be, when they could have been elsewhere:
“If you wanted to produce carbon and oxygen in roughly equal quantities by stellar nucleosynthesis, these are just the two levels you would have to fix, and your fixing would have to be just about where these levels are actually found to be.”[ix]

Curiously, this entire process relies on the fine-tuning of at least two more fundamental factors. The first is the mass of quarks, the tiny constituents of protons and neutrons. If their mass was larger by just one part in 1023 relative to the Plank mass, the resonance levels or carbon and oxygen would be disrupted. Neither would be produced.
The next crucial factor is the strength of the strong nuclear force, which holds protons and neutrons together in the nucleus. If it is increased by just 0.4%, it would disrupt the necessary resonance levels, and oxygen production would rapidly decline. We would have carbon, but no oxygen. If the strong nuclear force was diminished by just 0.4%, the opposite occurs. Abundant oxygen, but no carbon.
The strong nuclear force has yet another crucial role in the production of life-permitting chemistry. The strength of this force deeply affects the conversion of hydrogen to helium in stars. Helium production is important because it is the key link that allows the production of heavier elements like carbon, oxygen and iron. As we have seen, these heavier elements are seeded across the universe when stars explode in supernovae.
The strong force’s value is denoted by the letter ε [epsilon]. ε’s value is 0.007. If it were slightly weaker, at 0.006, helium would not be produced. No higher elements would be formed, and no planets would form. If the strong force were slightly stronger, at 0.008, then the production of helium would be too efficient. Stars would burn through all their hydrogen quickly. No hydrogen would be left to form water, H20.
From these we can see that the basis of all chemistry is rooted in the fine-tuning of the physics of our universe. Without this chemistry, there would be no biology.
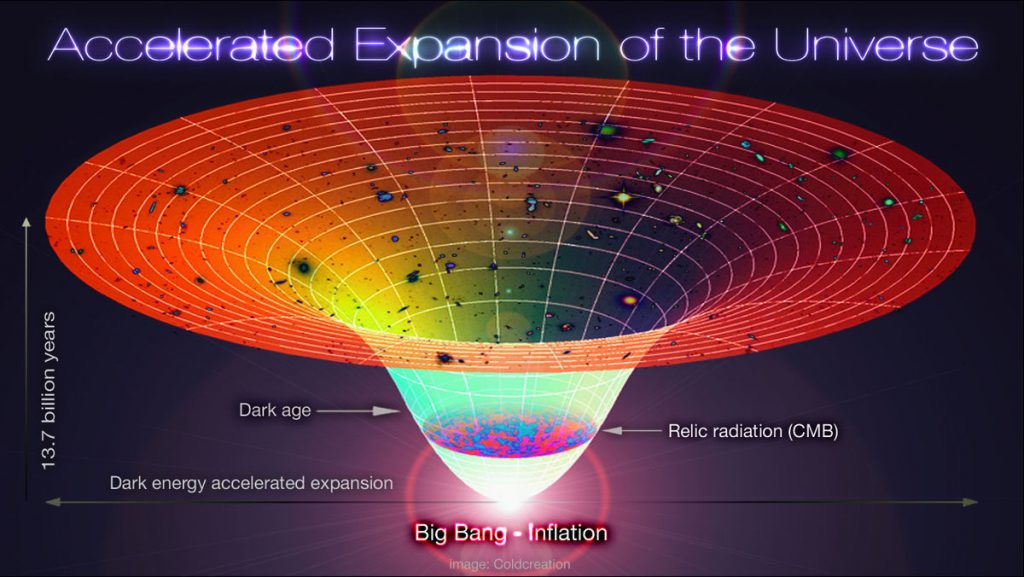
Cosmological Constant
There is one final constant that deserves special mention. It is known as the cosmological constant, and its discovery shook the world of science.
By the end of the 20th century, it was well accepted that the universe was expanding. Conventional wisdom told us that this expansion should be slowing down – dark matter should be exerting a gravitational pull on the universe around it, effectively applying the brakes to the expansion.
In the 1990’s however, it was found that the universe’s expansion was accelerating. Instead of slowing down, something was putting the universe’s pedal to the metal. The expansion was down to what we now term dark energy. This dark energy must be applying a repulsive force to overcome the universe’s gravity, a kind of anti-gravity.
The strength of dark energy’s ‘anti-gravity’ is governed by what physicists have termed the cosmological constant. If it were slightly stronger, then the universe would expand too fast – galaxies would not have the chance to form, meaning no stars, no planets, and no life. But if the repulsion was too weak, then gravity would have won out by now. The universe would have collapsed back into itself, and no progress would be made.
How finely-tuned is this repulsive force? Extraordinarily so, it seems. It started when physicists added up the energy of quantum fields, to see how much energy was in ‘empty’ space. They got an answer of 1093, an absurdly large mass density for a little centimetre! They then thought that there must be some other field which they hadn’t measured which was cancelling out that figure with a negative force, to equal zero.
But then it all went wrong. They discovered that empty space had a mass density of 10-28 grams per cubic centimetre. This means that something was cancelling out around 10119 worth of mass density, and leaving just a tiny bit – the exact amount needed to make sure the universe expands in a stable manner! Up or down by a factor of ten, and all would be lost.
Paul Davies, the acclaimed physicists and science-communicator, writes:
“A factor of ten may seem like a wide margin, but one power of ten on a scale of 120 is a pretty close call. The cliché that “life is balanced on a knife-edge” is a staggering understatement in this case: no knife in the universe could have an edge that fine. Logically, it is possible that the laws of physics conspire to create an almost but not quite perfect. cancellation. But then it would be an extraordinary coincidence that that level of cancellation—119 powers of ten, after all—just happened by chance to be what is needed to bring about a universe fit for life. How much chance can we buy in a scientific explanation?” [x]
Luke A. Barnes, a specialist in fine-tuning, summarises the issue:
“There is some reason — some physical reason — why the large contributions to the vacuum energy of the universe don’t make it life-prohibiting… This is fine-tuning par excellence.” [xi]
Conclusion
There is now a near-universal consensus amongst physicists that fine-tuning is real. The evidence shows that life can only exist if the universe inhabits a tiny intersection between finely-tuned initial conditions, the right physical laws, and finely-tuned physical constants. The most infinitesimal tweaks in any of these domains would produce dead universes. Such sterile universes, according to our modelling, are entirely physically possible; they vastly outnumber universes that can sustain life; and yet here we are. We must ask why. The universe must have been produced by something, what physicists often refer to as a ‘universe-generating mechanism.’ That something seems to have cared about producing life.
Sound familiar?
“Blessed is He in Whose hand is the kingdom, and He has power over all things; Who has created death and life that He might try you — which of you is best in deeds; and He is the Mighty, the Most Forgiving.
Who has created seven heavens in harmony. No incongruity canst thou see in the creation of the Gracious God.
Then look again: Seest thou any flaw? Aye, look again, and yet again, thy sight will only return unto thee confused and fatigued.”
Holy Qur’an, 67: 2-5
In Part 2, we will examine a range of responses to the fine-tuning of the universe, especially the multiverse, and argue that Divine Design is the only real solution to this enigma.
References
[i] Barnes, L. (2012). The Fine-Tuning of the Universe for Intelligent Life. Publications of the Astronomical Society of Australia, 29(04), pp.529-564. Section: A.1.16
[ii] Ryden, B. (2003). Introduction to cosmology. San Francisco: Addison-Wesley, p.193.
[iii] Lewis, G. and Barnes, L. (2017). A Fortunate Universe: Life in a Finely Tuned Cosmos. Cambridge: Cambridge University Press, p.125.
[iv] Lewis, G. and Barnes, L. (2017). A Fortunate Universe: Life in a Finely Tuned Cosmos. Cambridge: Cambridge University Press, p.91-92.
[v] Rees, Martin. Just Six Numbers: The Deep Forces That Shape the Universe (SCIENCE MASTERS) (Kindle Location 494). Orion. Kindle Edition.
[vi] Lewis, G. and Barnes, L. (2017). A Fortunate Universe: Life in a Finely Tuned Cosmos. Cambridge: Cambridge University Press, Chapter 4.
[vii] Rees, Martin. Just Six Numbers: The Deep Forces That Shape the Universe (SCIENCE MASTERS) (Kindle Locations 799-800). Orion. Kindle Edition.
[viii] Lewis, G. and Barnes, L. (2017). A Fortunate Universe: Life in a Finely Tuned Cosmos. Cambridge: Cambridge University Press, p.118.
[ix] Hoyle, F. (1982). The Universe: Past and Present Reflections. Annual Review of Astronomy and Astrophysics, 20(1), p.12.
[x] Davies, P. (2008). The Goldilocks enigma; Chapter 8. Boston: Houghton Mifflin.
[xi] Barnes, L. (2012). The Fine-Tuning of the Universe for Intelligent Life. Publications of the Astronomical Society of Australia, 29(04), pp.529-564. Section: 4.6








Good post. I study one thing tougher on different blogs everyday. It should all the time be stimulating to learn content from different writers and practice slightly something from their store. I’d want to use some with the content material on my weblog whether you don’t mind. Natually I’ll give you a hyperlink on your internet blog. Thanks for sharing.
Feel free to post excerpts with credit and links. Thanks!